You are here: Home: Meet The Professors Vol. 1 2004: Case 1

- Patient underwent mastectomy in 1977, followed by CMF and one year of tamoxifen.
- While followed for rising liver function tests, attributed to simvastatin, patient developed fatigue and right upper quadrant pain.
- Ultrasound revealed multiple hepatic lesions.
- Biopsy of hepatic lesions revealed ductal carcinoma that was ER/PR-negative, HER2-positive (IHC 3+). Original pathology from 1977 was unavailable.
- Mammogram and clinical exam of the contralateral breast were negative.
 |
| Key discussion points: |
 |
|
Quality control in ER/PR and HER2 assays |
|
Selection of chemotherapy in combination with trastuzumab in patients with HER2-positive metastatic disease |
|
Endocrine therapy in patients with ER-positive, HER2-positive metastatic disease |
|
Duration of trastuzumab therapy |
 |
Monitoring cardiac function in patients on trastuzumab |
 |
DR BEDNAR: This 67-year-old woman was originally taken care of by a physician who later retired from our practice. In 1977, the patient had a left mastectomy and then received CMF. She also took tamoxifen for about a year, and had not seen an oncologist in a long time.
Over the years, she developed hypertension and hypercholesterolemia. A couple of years ago she started taking simvastatin and then began to develop rising liver function tests. These were attributed to the simvastatin and she was simply followed. The patient then developed fatigue and right upper quadrant pain. An ultrasound demonstrated multiple hepatic lesions. Biopsy revealed ductal-type cells that were ER/PR-negative and IHC 3+ for HER2. Mammogram and physical examination of the contralateral breast were both negative.
DR LOVE: Were you able to obtain the original pathology from 1977 and compare it to the liver biopsy specimen?
DR BEDNAR: No, I was not.
DR LOVE: Harold, can you share with us how you would think through this patient's situation.
DR BURSTEIN: Well, the whole thing doesn't make much sense. It's not inconceivable that these metastases are from the original tumor, but it is certainly unusual. I would consider FISH in this situation, especially with a liver needle biopsy specimen.
DR VOGEL: I would send samples for estrogen receptor assay to two different reference labs, and I would be sure to obtain a FISH. One of the best correlates we have with hormone receptor-positive disease is disease-free interval. I published a paper in the 1980s on the response of tamoxifen in ER-negative disease. We had about a 25 percent response rate, and most of that was due to false-negatives. I would make absolutely certain that both of these assays were accurate before making any kind of decision with regard to treatment.
DR BILSKY: But even if the ER/PR receptors were positive and FISH confirmed HER2 overexpression, in someone with visceral disease, you'd be hard pressed to give hormonal therapy anyway.
DR VOGEL: Correct. If she had less severe liver problems and wasn't particularly symptomatic, I would probably try hormonal therapy despite the receptor negativity. I still want to know the receptor status because ultimately her cancer will recur and it would be nice to have hormones to fall back on.
DR LOVE: Chuck, assuming she is both FISH-positive and ER-negative, what specific systemic regimen would you recommend?

DR VOGEL: All of the chemo-trastuzumab regimens produce excellent response rates between 60 and 70 percent. We're going to need an adjuvant-like trial to produce sufficient power to prove that one regimen is superior to another. Outside of the context of a clinical trial, you can take your pick of weekly paclitaxel, weekly docetaxel, gemcitabine, vinorelbine or carboplatin/paclitaxel in combination with trastuzumab. I make the decision on the basis of toxicity after counseling and discussion with the patient.
DR LOVE: If this patient had an ER-positive, HER2-positive tumor, how might your approach change?
DR BURSTEIN: In a symptomatic patient like this one, I would probably induce her with chemotherapy and trastuzumab and then hopefully consolidate with hormone therapy later. In someone with minimally symptomatic metastatic disease, I would certainly feel comfortable starting with and obtaining as much mileage as I could out of endocrine therapy before pulling the chemotherapy/trastuzumab trigger.
There's no data in that setting to tell us whether continuing trastuzumab after stopping chemotherapy and adding hormones is clinically beneficial or not. In clinical practice, it is frequently difficult to get patients to discontinue trastuzumab, especially if they've had a good clinical response. I am comfortable giving a treatment break or switching to an every three-week schedule and adding an endocrine agent with trastuzumab.
DR VOGEL: Let me just make one other point because it is controversial. Until the ongoing clinical trials show unequivocal benefit of combining trastuzumab with endocrine therapy, I will treat HER2-positive, ER-positive patients with minimal disease with hormones up front without trastuzumab; however, Steve Jones, Mark Pegram and I agree to disagree on this topic. They use combination trastuzumab and hormonal therapy in these patients.
DR BURSTEIN: I'll just add that in the subset analysis of the pivotal trastuzumab trial, prior hormonal therapy did not adversely affect the outcomes with chemotherapy and trastuzumab together. For that reason, as does Chuck, I feel very comfortable offering endocrine therapy without trastuzumab, as long as it's clinically indicated, and then bringing in the trastuzumab later.
Many clinical trials are evaluating aromatase inhibitors plus or minus trastuzumab. Everyone expects to increase the response rate and time to progression by adding the trastuzumab early because it's an active drug in and of itself.
However, I assume that these studies are never going to answer whether or not there will be a survival benefit because they are relatively small and weren't designed to have that much follow-up. I think this question will be on the table for a long time.

 |
| Case follow-up: |
 |
| • |
Patient was treated with weekly paclitaxel/trastuzumab. |
| • |
Right, upper quadrant pain and smaller hepatic lesions resolved, dominant lesion was reduced by 50 percent and fatigue improved. |
| • |
After six months, treatment was discontinued. |
| • |
Three months later, CAT scan revealed that the dominant hepatic lesion had increased in size and smaller lesions redeveloped. CA 27-29 increased 15 to 20 points. |
| • |
Treated with trastuzumab monotherapy every three weeks. |
| • |
Eighteen months later, the patient was doing well with no toxicity and hepatic lesions shrinking on CAT. |
|
DR LOVE: Dr Bednar, what happened with this woman?
DR BEDNAR: I gave her weekly paclitaxel and trastuzumab and she actually did quite well. Her smaller hepatic lesions resolved, and the dominant one was reduced by over 50 percent. Her right upper quadrant discomfort also resolved and she felt generally improved. By the end of six months, she was starting to feel some fatigue and some cumulative chemotherapy effects. I was treating her weekly, giving her some weeks off here and there, but she had two children who lived in another state and she wanted to travel and not come in as frequently. I decided to give her a treatment break and I stopped treating her altogether.
DR GREENBERG: Did you stop the trastuzumab?
DR BEDNAR: I stopped everything and I followed her with CAT scans and checks of her CA 27-29 levels which were initially over 100 but then normalized. About three months later, on routine follow-up, the CT scan revealed that the dominant hepatic nodule started to increase in size, some other small nodules redeveloped and her CA 27-29 went up about 15 to 20 points.
Symptomatically, she was still fine, but I started her on trastuzumab alone every three weeks. She's been on that for about a year and half now, and she continues to do very well. I did another CAT scan about a month ago and her lesions continue to shrink. Symptomatically, she's doing very well and has no toxicity.
DR LOVE: Chuck, what's your usual approach to the patient who's responding to chemotherapy and trastuzumab in whom you want to discontinue the chemotherapy? Do you continue the trastuzumab?
DR VOGEL: Yes, we continue the trastuzumab, and we usually give it every three weeks. At the point at which they then progress on trastuzumab alone, we either reinstitute the original chemotherapy or switch to a different one along with the trastuzumab. This patient never failed the original chemotherapy so it would be reasonable to go back to that.
DR LOWENTHAL: When you are maintaining a patient for a long time on trastuzumab and she is doing well and has no symptomatic cardiac disease, how often do you do surveillance MUGAs?
DR BURSTEIN: We looked at that issue in one of our trastuzumab and vinorelbine trials. We did a baseline MUGA and then a follow-up at 16 weeks. Among those patients who had preserved left ventricular ejection fraction (LVEF) of 50 percent or greater at 16 weeks, none of them went on to subsequently develop symptoms of heart failure or significant declines in LVEF.
By contrast, in two of the patients who had declines in LVEF at 16 weeks, we saw problems. One actually developed heart failure and the other had a drop in ejection fraction to about 40 percent. While this data only applies to that specific regimen, this has become our routine algorithm.
Anecdotally, I have not seen any late-onset heart failure or changes in LVEF once patients get past the first few months of trastuzumab-based therapy. In my experience, cardiac changes usually occur in the first two or three months of therapy, so I think if you recheck the MUGA around three and four months and the patient is clinically stable, you don't need to frequently check it again.
DR LIPSHUTZ: From a practical standpoint, when you see a patient's ejection fraction fall, I assume you withhold trastuzumab. I also assume that because this is metastatic disease that's responding to trastuzumab, you have a strong desire to resume the trastuzumab after the ejection fraction has improved. What has happened to patients for whom you've employed that strategy?
DR BURSTEIN: Well, fortunately, there aren't many patients like this. Even the incidence of asymptomatic declines in LVEF, in our experience with taxanes or vinca-based therapies, is much less than five percent. For this type of case, I typically stop the trastuzumab, have the patient see a cardiologist, work on optimizing her hemodynamics, and then see what happens with her cancer using other non-trastuzumab-based approaches like hormones or other chemotherapy. At some point, if her EF has recovered, and it certainly may, I will rechallenge her with trastuzumab if the clinical circumstances demand.
I have heard anecdotes of physicians treating through asymptomatic LVEFs in the thirties, and that the LVEF will come back over time once you stop the trastuzumab. I don't know that you can authoritatively say that it's always reversible, so that's just not something I've done. When a patient's LVEF gets below 45 or 50 percent, I get more squeamish.
DR LOVE: Chuck, what's your approach to cardiac monitoring and dealing with drops in ejection fractions?
DR VOGEL: We've actually just adopted a policy of doing MUGAs every six months for a couple of years. As far as treating through, we don't worry too much until the LVEF reaches about 40 percent in a responding patient. If you have somebody with liver metastases that is well controlled but she also has a slowly dropping ejection fraction, it's a good idea to do echocardiograms more frequently and keep her on trastuzumab as long as she's working closely with the cardiologist.
DR BURSTEIN: Chuck, could you elaborate on that? I have not seen patients in whom the LVEF drops steadily from 62 to 57 to 52 to 48 to 43. In my experience, patients are doing well, doing well, doing well, and then the LVEF just drops. By contrast, I can't think of any patient on trastuzumab in whom I've seen a drop after six months. Have you seen those cases?
DR VOGEL: Yes, I had one patient who was on trastuzumab for six years when she developed clinical congestive cardiac failure. She was so far out that we weren't doing routine MUGAs.
DR FRIEDBERG: Harold, is it true that patients treated with prior anthracyclines and then trastuzumab do not recover cardiac function as quickly?
DR BURSTEIN: I don't know enough about cardiac toxicity to say that, but I've certainly had patients who have received prior anthracyclines - either in the adjuvant or metastatic setting - and have developed heart failure while taking trastuzumab. Some of those patients have experienced lingering problems with heart failure. I don't know if we have a pure enough population of anthracycline-naïve patients who have had trastuzumab-related cardiomyopathy and then got better to truly answer that question.
DR VOGEL: That's really the issue. When you start talking about people who haven't recovered, I think it's very difficult to differentiate between the anthracycline cardiotoxicity and the additional toxicity of the trastuzumab.
DR BURSTEIN: One of the things that Andy Seidman found when he did his comprehensive review of cardiac toxicity of trastuzumab was that prior anthracycline exposure was clearly a risk factor. Some data indicate that trastuzumab may actually interfere with the heart's normal myocyte repair, so adding an anthracycline hit to the trastuzumab may produce a particularly vulnerable situation. How that plays out in the clinical setting is really not known.
DR LOVE: I'm curious, Dr Bednar, has the experience with this patient in any way changed your algorithm or approach to management of HER2-positive breast cancers?
DR BEDNAR: Yes. I think perhaps, after induction with chemotherapy and trastuzumab, I might keep patients on trastuzumab as maintenance.
DR LOVE: It's interesting how sometimes one patient or one experience will have more effect on us than reading all the literature in the world.
It reminds me a little bit about the early days of adjuvant tamoxifen when we treated patients for a year or two and we saw women coming back and then relapsing. Same situation: We re-treated them and they responded. You only had to see one patient like that to start thinking about using tamoxifen for a little longer.

"A large randomized phase III pivotal trial comparing chemotherapy alone versus chemotherapy plus trastuzumab showed that the addition of trastuzumab improved objective response rates, median duration of response, and overall survival. Unfortunately, myocardial dysfunction was markedly increased in the patients who were receiving concurrent anthracycline-based therapy.. When trastuzumab was approved by the US Food and Drug Administration in September 1998 for the treatment of women with metastatic breast cancer whose tumors overexpress HER2, it was approved as a single agent in the salvage setting or as first-line treatment in combination with paclitaxel. It is universally accepted that trastuzumab is not recommended in combination with anthracyclines. An independent Cardiac Review and Evaluation Committee reported on several trastuzumab trials. The findings supported the observations that trastuzumab was associated with an increased risk of cardiac toxicity, and age was associated with increased risk in the anthracycline-treated subset. It is important to note that in the pivotal trial, the subset treated with trastuzumab and anthracyclines still had a treatment advantage with an improved response rate (64.9% v 42.1%) and a survival benefit."
 |
| SOURCE: Theodoulou M, Seidman AD. Cardiac effects of adjuvant therapy for early breast cancer. Semin Oncol 2003;30(6):730-9. Abstract |
 |
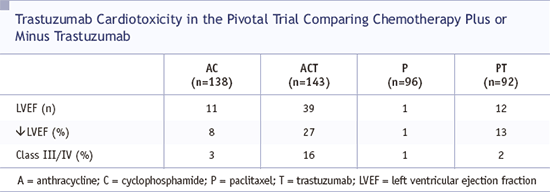 |
| SOURCE: Theodoulou M, Seidman AD. Cardiac effects of adjuvant therapy for early breast cancer. Semin Oncol 2003;30(6):730-9. Abstract |
 |
Select publications
|
