You are here: Home: Meet The Professors Vol. 3 2004: Case 1

- Presented with a palpable lesion in her left breast
- Mammography revealed 1.7-centimeter, 1.5-centimeter and 0.8-centimeter lesions in the same quadrant
- Underwent a modified radical mastectomy
- SLNB-positive, with 12/14 axillary lymph nodes involved
- ER was 10 percent; PR was negative
- HER2-positive via FISH
- Metastatic workup including a normal bone scan, CT scan of the chest and abdomen, and echocardiogram
 |
| Key discussion points: |
 |
|
Protocol and nonprotocol options for adjuvant trastuzumab |
|
Role of adjuvant ovarian ablation in addition to tamoxifen or an aromatase inhibitor in premenopausal patients |
|
Use and selection of growth factor support |
|
Switching patients from tamoxifen to an aromatase inhibitor |
 |
DR ANSARI: This 38-year-old premenopausal woman presented with a palpable lesion in her left breast. Mammography demonstrated three lesions in the same quadrant: 1.7- centimeter, 1.5-centimeter and 0.8-centimeter.
She underwent a modified radical mastectomy, with sentinel lymph node biopsy and complete axillary dissection. Twelve out of 14 axillary lymph nodes were positive for metastases. Her tumor was 10 percent ERpositive, PR-negative, and HER2 gene amplification by FISH was positive.
DR LOVE: Can you describe her lifestyle and life circumstances?
DR ANSARI: She was married and had three small children; the youngest was about six years old at the time of her diagnosis.
She was concerned about her appearance and after the mastectomy, she underwent immediate reconstruction, including a tummy tuck and flap. She was a manager of a store and wanted to maintain her appearance for her job.
DR LOVE: Adam, this is a very challenging case involving a young woman with 12 positive nodes. What are your thoughts?
DR BRUFSKY: We have clinical trials available for her, with trastuzumab in some of the arms. BCIRG trial 006 — AC/docetaxel, AC/docetaxel plus one year of trastuzumab, or carboplatin/docetaxel plus trastuzumab — would have been our first choice, but that trial just closed. At our institution we would offer her participation in NSABP-B- 31, which randomly assigns patients to AC followed by paclitaxel alone or with one year of trastuzumab.
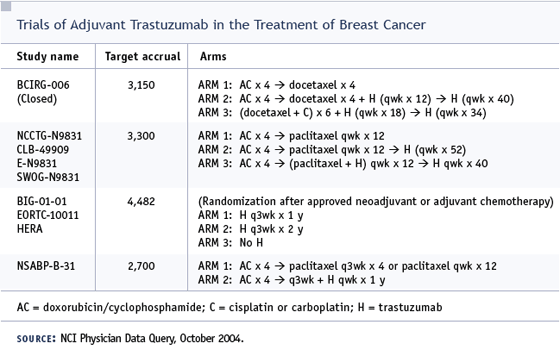
I would not utilize adjuvant trastuzumab in this woman off protocol because the data from NSABP-B-31 presented at the 2003 San Antonio Breast Cancer Symposium suggested that women receiving AC 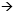 paclitaxel plus trastuzumab had a clinical congestive heart failure or cardiac death rate of approximately 3.5 percent. paclitaxel plus trastuzumab had a clinical congestive heart failure or cardiac death rate of approximately 3.5 percent.
I would probably administer a regimen containing AC and a taxane. The TAC regimen would be reasonable or AC followed by paclitaxel or docetaxel. I’ll leave the dose-dense issue for Dr Budman.
DR LOVE: What about endocrine therapy?
DR BRUFSKY: We consider her ER-positive. In fact, the most recent St Gallen consensus conference indicated that staining over one percent was considered positive, so she was clearly ER-positive. I would treat her with tamoxifen after the chemotherapy.
Whether to use ovarian ablation in addition to tamoxifen is a controversial question. No has yet shown ovarian ablation after chemotherapy provides any benefit.
DR LOVE: Dan, would you use trastuzumab? What type of chemotherapy? What type of hormonal therapy? Let’s assume she’s still actively menstruating after chemotherapy.
DR BUDMAN: This is a difficult case. I assume you’re going to stage her carefully, because having 12 positive nodes is almost tantamount to metastatic disease.
DR LOVE: Dr Ansari?
DR ANSARI: She had a metastatic workup done, which included a bone scan, CT scan of the chest and abdomen and an echocardiogram, and all were normal.
DR BUDMAN: For this woman we would strongly recommend Edith Perez’s study, NCCTG-9831, in which the patient has two out of three chances of receiving trastuzumab. The cardiac events are noted very carefully in that trial. Patients are randomly assigned to AC followed by paclitaxel with or without trastuzumab, or AC followed by paclitaxel followed by trastuzumab. If anybody needs this type of study, it’s this woman.
If that’s not possible, I would offer dosedense chemotherapy. In the New York area, we’ve been indoctrinated in dose-dense chemotherapy, and I think it’s a very reasonable therapy. More than 3,000 patients were enrolled in CALGB-9741, and the dose-dense schedule clearly offers a benefit.
DR LOVE: Dose-dense AC followed by paclitaxel or sequential single agents?
DR BUDMAN: It’s “dealer’s choice” whether you use AC followed by paclitaxel or sequential. In young women, I usually administer AC together followed by paclitaxel. The combination frequently causes anemia, but younger women tolerate it well and it’s over faster.
DR LOVE: Would you utilize filgrastim or pegfilgrastim?
DR BUDMAN: I use pegfilgrastim. Enough evolving data exist from the CHOP data, and we’ve never had a problem with pegfilgrastim. It makes quality of life better.
In terms of endocrine therapy, no evidence exists that castration following chemotherapy is effective. A very interesting trial that should be a very high priority is the SOFT trial. Women who remain premenopausal after chemotherapy receive tamoxifen, ovarian ablation with tamoxifen, or ovarian ablation with exemestane. The SOFT trial would also be a good choice for her, and it’s available through CTSU.
DR LOVE: What about using that strategy in a nonprotocol situation, with an LHRH agonist plus an aromatase inhibitor? This woman has an ER-positive, PgR-negative tumor. We had some interesting data presented from the ATAC trial at the 2003 San Antonio meeting showing a particularly dramatic decrease in relapse rate using anastrozole. Any thoughts about that?
DR BUDMAN: We don’t have the answer. We have no long-term data with aromatase inhibitors in young women. On the other hand, if anybody’s at high risk for relapse, it’s this woman.
DR WADE: I’d like to ask a question of Dr Budman or Dr Brufsky regarding equipoise in the Perez trial, recognizing that the standard arm is still the standard schedule of AC followed by paclitaxel. Another randomized trial shows a modest but measurable improvement with the dosedense utilization of those same drugs.
DR BUDMAN: In Edith’s trial, the taxane is administered weekly. It may be that giving the weekly taxane is better than administering it every two weeks. We don’t know that. At our institutions, we feel that it’s a very reasonable therapy to offer.
DR LOVE: Dr Dragon?
DR DRAGON: Jim Wade brought up a very interesting point that was highlighted by a patient I put on NSABP-B-31 last week. She is a public health professional with a PhD, and she knew as much about the ongoing clinical trials as most physicians.
When I offered her participation in NSABPB- 31 and we discussed the arms and the dose-dense chemotherapy option, she pointed out that the nontrastuzumab arm did not receive dose-dense chemotherapy.
Was this a reasonable trial in which to be enrolled? I pointed out to her that NSABPB- 31 gives the treating physician the choice of paclitaxel schedule — either every three weeks or every week, which we have to elect at the time of randomization.
I felt that was a justifiable adjustment in the schedule, which may be most of the benefit in the dose-dense regimen. The Seidman study in the metastatic setting clearly highlights that paclitaxel every 21 days for four cycles is probably not the best administration schedule, which we’ve known for a long time.
It is a point of equipoise. If we push dosedense chemotherapy to its limit, we will have to terminate most of the ongoing randomized clinical trials that have a standard treatment arm of AC followed by paclitaxel. It would be very disappointing to be in that position.
DR BERRY: Many patients don’t want to enroll in clinical trials because often the trials don’t address their concerns. With regard to targeted therapy, such as trastuzumab, I’ve actually taken the plunge and treated a number of women off study because they’ve had a horrible potential outcome like this woman.
I have given the taxanes in a dose-dense fashion along with the weekly trastuzumab until the taxane was finished, and then continued with trastuzumab every three weeks for a full year. Anecdotally, the three or four patients whom I’ve treated have been doing well.
It’s a matter of being honest with the patients and making sure they understand that what we’re doing is not a standard practice. We must make it clear that we are making a value judgment based on the risks.
We do not present it to women who are not willing to enroll in clinical trials. I’ve not run into any trouble with cardiotoxicity. I monitor the MUGA scan every six months, and it has not been an issue.
I question whether 12 months is the correct duration of trastuzumab, and, in fact, the HERA study is evaluating 12 versus 24 months of trastuzumab. That’s another question beyond whether you should use trastuzumab at all.
DR LOVE: Dan, in a woman who is still menstruating after chemotherapy, do you consider ovarian ablation an option? Do you consider ovarian ablation plus an aromatase inhibitor an option in this situation?
DR BUDMAN: No good data exist for ovarian ablation alone after chemotherapy. On the other hand, I believe the SOFT trial is the most interesting ongoing investigation and I would try to enroll that woman in it.
If she refuses I would have no objection to an aromatase inhibitor plus ovarian suppression or ablation because she has 12 positive nodes and we need to try everything possible.
DR DRAGON: I struggle over whether to offer adjuvant trastuzumab or not, because intuitively I have the sense it’s the right thing to do.
We’ve been down that road before with bone marrow transplant, and we don’t want to do that again. I would be reluctant to be drawn. We’re attempting to be driven by data, and using clinical trial data is important to us. I think we need to learn the lesson one time only.
DR LOVE: Adam, what does the Adjuvant! model say? This woman is going to receive a taxane-containing chemotherapy regimen and tamoxifen. What’s her risk of relapse and death?
DR BRUFSKY: Within 10 years, her risk of relapse is 88 to 89 percent with no therapy. AC followed by a taxane every three weeks followed by tamoxifen results in a risk reduction of about 42 percent.
DR LOVE: So she still has a residual relapse rate of 46 percent? Is anyone using endocrine therapy other than tamoxifen alone in this situation?
DR MERKEL: Outside of the constraints of NSABP-B-31, which would be my first choice, I would actually push for ovarian ablation with an aromatase inhibitor, because this HER2-positive tumor is the tumor phenotype for which I would least trust tamoxifen to provide any benefit.
DR LOVE: If she is still menstruating at the end of chemotherapy, you would use an LHRH agonist plus an aromatase inhibitor?
DR MERKEL: Yes.
DR NABHAN: Approximately 10 years ago, it would have been considered cruel not to offer a bone marrow transplant. We’re being careful and not even offering ovarian ablation and an aromatase inhibitor when the risk of dying of breast cancer is significantly high. I would tend to offer ovarian ablation in this setting.
DR BERRY: I agree with Dr Merkel. If I were seriously thinking about utilizing ovarian ablation for this woman, I’d give an aromatase inhibitor as the follow-through.
DR LOVE: Adam, the woman has an ERpositive, PgR-negative, HER2-positive tumor. Are you more inclined to consider an LHRH agonist plus an aromatase inhibitor?
DR BRUFSKY: Before listening to the discussion, I wasn’t thinking about an aromatase inhibitor, but I think the downside risk is not that high.
This woman’s major cause of mortality in the next 10 years is breast cancer, and I’d probably want to be as aggressive as possible, understanding that we don’t know the long-term cognitive effects of aromatase inhibitors in young women.
In her case an aromatase inhibitor is probably superior, at least in the postmenopausal setting, so I have no problem giving this woman an aromatase inhibitor and an LHRH agonist.
Before the NSABP-B-31 data came out, I treated 10 to 20 patients like this woman with trastuzumab off study. A few have had their ejection fractions go down.
Since B-31, I’ve stopped that, but two or three years from now when the data is complete, the docetaxel/carboplatin/trastuzumab arm of BCIRG-006 may become the standard of care for these women. That’s something to consider if you’re thinking of off-study therapy.
DR LOVE: Dr Ansari, can you give us a follow-up on what happened with this woman?
DR ANSARI: This lady was diagnosed in October 2001, so some of the trials and trial results we’re talking about were not available at that time.
When I saw her, we had two trials available: BCIRG-006 and NSABP-B-31. The patient chose our BCIRG trial. According to that trial, her HER2 slides had to be sent to UCLA, and the results were available in five days. When we received the results, her HER2 was negative at UCLA, so she didn’t qualify for that trial. She was treated outside the clinical trial with doxorubicin and cyclophosphamide every two weeks for four cycles with growth factor support followed by paclitaxel in a three-hour infusion every two weeks for four cycles. This was before the dose-dense regimen was published, but she did receive a dose-dense regimen.
At the conclusion of her chemotherapy, she was still menstruating and I started her on tamoxifen and goserelin. Her last follow-up visit with me was about three weeks ago and she is still on goserelin and tamoxifen. She’s been receiving risedronate because her yearly bone mineral density assessment revealed some bone loss, but her scans are normal right now.
DR LOVE: I was fascinated by the fact that this woman received dose-dense AC 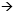 T before the 9741 trial was reported. I thought Dr Ansari had psychic abilities. Maybe you can talk a little bit about how this woman ended up receiving that therapy. T before the 9741 trial was reported. I thought Dr Ansari had psychic abilities. Maybe you can talk a little bit about how this woman ended up receiving that therapy.
DR ANSARI: At that time, we were impressed by the data from Memorial Sloan- Kettering on the dose-dense regimen, even though it was not a randomized trial. We thought if we want to give her the most benefit outside of a clinical trial, maybe the dose-dense regimen would be appropriate.
She tolerated it very well. Since then we’ve had a lot of experience administering dosedense chemotherapy, especially in patients with multiple positive lymph nodes. My experience is that with growth factor support — and we almost universally use pegfilgrastim — they tolerate therapy very well — probably better than with the every three-week schedule. Today, I would definitely offer these patients participation in B-31.
DR LOVE: Dan, a theme of this whole discussion is, “What are reasonable, evidencebased options to consider?” In this compelling situation involving a young mother with a poor prognosis after standard therapy, is trastuzumab an option. It was a falsepositive HER2, so trastuzumab is not an option. Another issue of dose-dense chemotherapy arose before the data was published.
As risk increases and age decreases, do you see people leaning more toward therapies that aren’t fully proven? What are your thoughts about the approach to adjuvant therapy from that perspective?
DR BUDMAN: Well, you’re “damned if you do and damned if you don’t.” Unfortunately, we have a fair idea of what the biology of this disease is, even though we don’t know how to treat it well. Without trying to be conservative I always worry that we’ve gone down lots of paths before with a lot of different types of tumors and said, “This is the answer,” and then it turns out that it really offers no major benefit.
It’s nice to see that a winner can be picked out now and then. On the other hand, we do not have mature data. We’ve been wrong before, and we could be wrong again.
I would have pushed this woman as hard as I could to participate in a clinical trial. We desperately need to finish these trials in real time, which we’re not doing.
DR LOVE: Any final comments on this case?
DR ANSARI: She has had three years of tamoxifen, and she’s on goserelin. She is about 42 years old. New data coming out indicate that two to three years of tamoxifen followed by an aromatase inhibitor looks better than continuation of tamoxifen: Is it time to discontinue tamoxifen and put her on an aromatase inhibitor?
DR LOVE: You’re talking about the switching issue, but this is a woman who’s been made menopausal by an LHRH agonist. We’re really getting out there a little bit. Adam?
DR BRUFSKY: What about convincing her to undergo an oophorectomy and then putting her on an aromatase inhibitor?
DR LOVE: Do you feel better about oophorectomy than just keeping her on goserelin?
DR BRUFSKY: Yeah, I do. It’s more permanent. I’ve had women who were borderline suppressed in whom I’ve given an aromatase inhibitor and they actually began to menstruate again.
Aromatase inhibitors inhibit peripheral aromatase, so feedback on the ovaries is lost. Although the LHRH agonist causes suppression, in certain rare cases that may be overcome and menstruation resumes. For example, I’ve treated women who were perimenopausal from their chemotherapy who began to menstruate when switched to an aromatase inhibitor.
DR LOVE: So what would you do after the oophorectomy?
DR BRUFSKY: I would offer her an aromatase inhibitor.
DR LOVE: Would you have done that up front if she had an oophorectomy?
DR BRUFSKY: Yes.
DR LOVE: Dan?
DR BUDMAN: I probably would have given her tamoxifen in that setting. In light of her high risk, I’m not averse to giving her an aromatase inhibitor, as long as she is aware that we really don’t know how it’s going to affect a 40-year-old woman 20 years later.
DR LOVE: She’s been on tamoxifen for three years. Would you switch her to an aromatase inhibitor?
DR BUDMAN: At that point, I would have done an oophorectomy because of qualityof- life issues. She doesn’t have to worry about shots or whether she’s going to break through on the LHRH agonist. Oophorectomy is eminently reasonable, and I’d probably give her an aromatase inhibitor as long as she’s willing to accept the risk.
DR LOVE: Adam, the whole thought process has changed in the last year with the MA17 data evaluating letrozole after five years of tamoxifen. Now two trials have evaluated aromatase inhibitors after two or three years of tamoxifen — one with anastrozole, the other with exemestane.
The patient’s residual risk over time becomes an important issue. This woman has 12 positive nodes, and she’s made it three years free of disease. How much more residual risk of relapse does she have now? What would it be in five years, seven years, 10 years, and how does that factor into your decisionmaking?
DR BRUFSKY: I still think the residual risk with 12 nodes positive is substantial — even up to 10 years — and she would be a perfect candidate for MA17. She would definitely benefit from continued aromatase inhibition for five more years.
DR LOVE:Dan, any predictions about her relapse rate over time if she just continued with goserelin and tamoxifen for five years? How would that affect her risk for relapse?
DR BUDMAN: For patients with ER-positive disease, late relapses are not uncommon. The concern with the MA17 data is whether ER-positive patients with the best prognosis were selected because they are more likely to survive five years. That’s a different issue because if this patient survives five years, I would be worried that her relapse rate would still be substantial because of her significant nodal status.
Select publications
|
