You are here: Home: Meet The Professors Vol. 3 2004: Case 3

- Presented at 44 years of age with 1.8-centimeter primary tumor that was ER/PRpositive, HER2-positive (3+ by IHC)
- Four out of 31 lymph nodes were positive
- Treated with lumpectomy, adjuvant AC x 4 and radiation therapy
- Menstruation ceased after chemotherapy
- Treated with adjuvant tamoxifen
 |
| Key discussion points: |
 |
|
Role of ovarian ablation in premenopausal patients at high risk |
|
Switching to an aromatase inhibitor after three years of tamoxifen versus up-front use of adjuvant aromatase inhibitors |
|
Selection of hormonal therapy for the patient with HER2-positive disease |
|
Choice of adjuvant chemotherapy for the patient with positive lymph nodes |
 |
DR TSARWHAS: This woman presented a few years ago. At that time, she was in her midforties and had Stage II breast cancer. Her primary tumor was 1.8 centimeters, ER/PRpositive and HER2-positive (3+ by IHC). She had four out of 31 lymph nodes involved. She was premenopausal and was treated with lumpectomy.
DR LOVE: Hy, if she presented today, how would you think through her therapy?
DR MUSS: I would try to enroll her in a clinical trial, but if she wasn’t interested, several options for chemotherapy. Dosedense AC 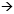 T or TAC are leading the pack in terms of the data, so I would pick one of those two regimens. T or TAC are leading the pack in terms of the data, so I would pick one of those two regimens.
She would undergo breast irradiation and then I would try to enroll her in the SOFT trial. SOFT is for premenopausal women who maintain their menses, and the randomization is tamoxifen, tamoxifen/ovarian ablation, or ovarian ablation and an aromatase inhibitor. I think it’s a very good trial because it’s trying to answer some of the questions that we all want answered.
In a patient with four positive nodes, I would probably recommend ovarian ablation and either put her on tamoxifen or an aromatase inhibitor after discussing the pros and cons. Tamoxifen alone would also be perfectly reasonable. I would not put her on trastuzumab outside of a clinical trial, even though her tumor is IHC 3+.
I think the ovarian ablation data is going to eventually show that it helps a little bit, but I don’t think it is going to be any type of “home run.” However, in someone with four positive nodes and a tumor like this, I would lean toward making the jump. I really don’t think it is going to have a drastic effect on quality of life in the long run, but you might give her a few percent edge.
DR LOVE: Does the HER2 status affect your choice of hormonal therapy?
DR MUSS: I would say it does. Compelling preclinical data indicate that HER2 is involved in phosphorylating receptors and activating them even without a ligand and bypassing the effects of drugs like tamoxifen. Clinical data from trials in metastatic disease show patients with HER2-positive disease are slightly more resistant to endocrine therapy, whether it’s an aromatase inhibitor or tamoxifen. An aromatase inhibitor may be a better choice up front in patients with HER2-positive disease.
DR LOVE: Tom, how would you have approached this woman?
DR BUDD: At that time, I would have used an anthracycline/taxane-containing regimen. For the hormonal regimen, the thought that HER2 positivity might be a reason to think about something other than tamoxifen is interesting, but for standard clinical practice I would probably use tamoxifen alone as the standard of care.
The combination of ovarian ablation and tamoxifen has never been compared to tamoxifen alone, so we don’t have a true comparison. Clearly, tamoxifen and chemotherapy act independently and add to each other’s effects, so the question is whether ovarian ablation adds to that — and we just don’t know the answer.
From the Intergroup trial in patients at lower risk who were randomly assigned to ovarian ablation plus tamoxifen versus tamoxifen alone, we know that ovarian ablation adds toxicity. For that reason, I tend to use tamoxifen alone as adjuvant treatment, and even though the HER2 positivity worries me a bit, I think I would go ahead with tamoxifen.
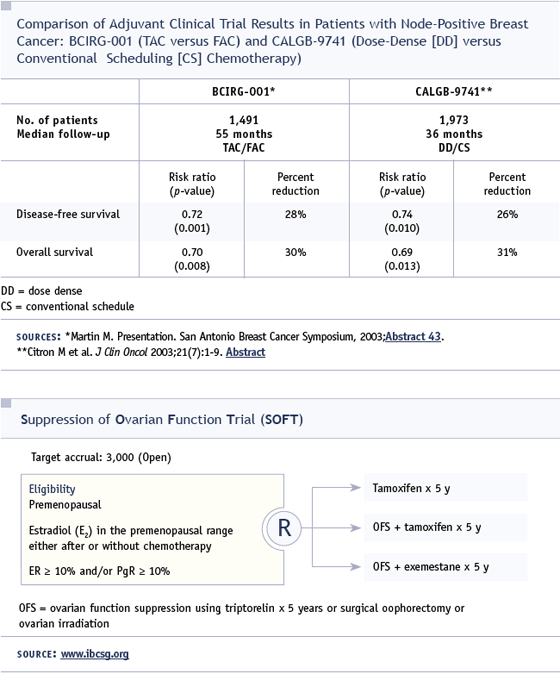
DR LOVE: You’re the principal investigator of a new Intergroup SWOG study that is a follow-up to the CALGB-9741 dose-dense study — can you tell us about the design of that trial and its rationale?
DR BUDD: Dose-dense therapy seems to be the optimal way to administer the combination of AC/paclitaxel. If that’s the anthracycline/ taxane-containing regimen you’re using, I would tend to use dose-dense therapy in most cases.
SWOG-S0221 is based on the dose-dense CALGB-9741 trial, some SWOG studies and some studies done at the University of Washington evaluating a different regimen of doxorubicin/cyclophosphamide. In that regimen, cyclophosphamide is given orally continuously for 15 weeks, and doxorubicin is given weekly. To maintain the dose, it’s necessary to give G-CSF every day except on the days of intravenous drug administration.
This regimen produced very promising results in a pilot adjuvant trial and a pathologic complete response rate of approximately 24 percent in locally advanced disease in the Southwest Oncology Group. Based on those preliminary data, that regimen is being compared to dose-dense AC given for six weeks in order to compare equivalent durations of therapy — 15 weeks versus 12 weeks. It also has a randomization between dose-dense paclitaxel every two weeks for six cycles and weekly paclitaxel for 12 weeks.
DR LOVE: What’s the growth factor support being used in this study?
DR BUDD: For the every two-week treatments, it’s pegfilgrastim, and for the daily and weekly treatments, it is filgrastim.
DR LOVE: What happened to this patient, Dr Tsarwhas?
DR TSARWHAS: This patient originally presented at approximately the same time that CALGB-9344 was presented, and at that point we discussed the benefit of a taxane in patients who were ER-positive and went on to receive tamoxifen. In fact, one of our thought leaders in Chicago was not recommending the addition of a taxane in that setting.
Obviously, thinking has changed over the years, but at that point I treated her with four cycles of AC. She stopped menstruating after chemotherapy and went on to receive adjuvant radiation therapy. She then started on tamoxifen.
She did well for a couple of years, but recently came to the office with some complaints of back pain. My initial thought was, “She’s three years out now. She didn’t received a taxane. She was IHC 3+ for HER2 overexpression and I have her on tamoxifen. I missed my chance, and her cancer has recurred.”
We did a bone scan and an MRI. I don’t routinely check markers, but if a patient has symptoms, I check them and I did in this case. Everything came out normal. She had some arthritis in her lower back so I prescribed 25 mg of rofecoxib and her symptoms improved immediately.
Reviewing this case again prompted me to bring up the idea of switching to an aromatase inhibitor, as I now discuss it as an option with any patient who is either on tamoxifen or completing tamoxifen. She is an intelligent patient and we discussed the fact that her tumor was HER2-positive, she didn’t receive a taxane and maybe she would benefit from switching. Given both our concerns, we made the switch from tamoxifen to exemestane.
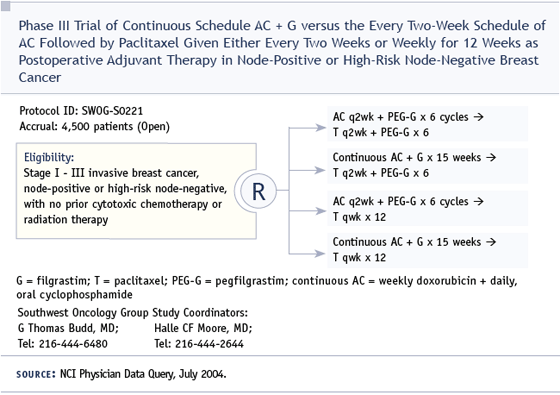
DR LOVE: Let’s talk about the continuum of hormonal therapy. I think we’re all much more sensitive to it with the emergence of these switching reports. Hy, how do you approach patients who are in their first five years of adjuvant tamoxifen? Do you routinely bring up the issue of switching? How do you determine when you’re going to switch therapies?
DR MUSS: This is a work in progress and my views have changed a lot this year, based on the fact that now we have several trials — ATAC, the Intergroup exemestane trial, the NCIC trial with letrozole after five years of tamoxifen and a smaller Italian trial — all showing the relative superiority of the aromatase inhibitors.
I thought Dr Goss’ presentation of the letrozole trial at ASCO was very impressive. Even with all the design issues, with distant metastases as the initial endpoint, patients with positive nodes have a statistically significant survival advantage with letrozole. I have to believe that the exemestane study, which actually had more events than the initial letrozole trial, will probably also show a survival advantage, and I have been discussing the aromatase inhibitor data with the majority of my patients.
In this patient with four positive nodes and HER2-positive disease, I would probably encourage her to switch to an aromatase inhibitor, and what I generally do is I pick the aromatase inhibitor that matches the patient’s status. This patient has had two to three years of tamoxifen, so I would use exemestane. If she had five years of tamoxifen, I would use letrozole, and if this was de novo disease, I would pick anastrozole. I think that is a reasonable approach, even though I don’t believe the three differ much biologically or in terms of overall effectiveness.
DR LOVE: One of the questions I’ve been asked since the switching data has emerged is, in the long run, would patients be better off having tamoxifen for two to three years or five years and then switching, as opposed to starting on an aromatase inhibitor up front?
DR MUSS: I don’t think it makes great sense to do that because in all of these studies the relapse rates and the rates of distant metastases have been higher in the tamoxifen arms. Whether you begin up front, in the middle or out back, the aromatase inhibitors are doing better than tamoxifen in every study. Mathematically, I think it is a better strategy to use your best first. Otherwise, you lose a few patients to distant relapse that you might have salvaged with the aromatase inhibitor.
DR TSARWHAS: How long do you leave a patient on the aromatase inhibitor if you switch in the middle?
DR MUSS: Great question. I don’t know the answer. I’m going to probably use it for five years provided no accelerated bone loss occurs.
Select publications
|
