You are here: Home: Meet The Professors Vol. 3 Issue 6 2005: Case 2

Edited excerpts from the discussion:
DR SMITH: My patient is a 46-year-old intramenopausal
woman with irregular periods.
She developed a 1.4-centimeter, Grade II
infiltrating ductal carcinoma, which was
ER-positive, PR-negative and HER2-positive
— IHC 3+ and amplification by FISH.
She had three positive lymph nodes and
was accepted into the Intergroup adjuvant
trastuzumab trial, but when randomly
assigned to the nontrastuzumab arm, she
insisted on receiving trastuzumab and
dropped out of the trial.
DR LOVE: Were you surprised that this
patient dropped out of the clinical trial?
DR SMITH: I was surprised and a little
annoyed, since it confounds the trial results.
Initially, I persuaded her to stay on study,
but then she sought other opinions until
she found someone who would put her on
trastuzumab and then returned to me. This
actually occurred about a month before the
adjuvant trastuzumab data were released
last April.
I administered four cycles of AC followed by
weekly paclitaxel with concomitant trastuzumab
and, upon conclusion of the paclitaxel,
continued trastuzumab every three
weeks. I also have her on tamoxifen.
DR LOVE: Interesting. Can you tell us a bit
more about this woman and her situation?
DR SMITH: The patient is married and has
two children. She is a psychiatrist and her
sister is a radiologist. As many of these
patients do, she went on the internet, made
the rounds, and carefully read the study
consent.
DR LOVE: Gersh, what are your thoughts
regarding this patient enrolling and then
dropping out of the clinical trial in order to
receive adjuvant trastuzumab?
DR LOCKER: I wish I could say that this
was an isolated phenomenon, but this is a
common problem. In every consent form it
states that patients can withdraw consent
at any time, and a lot of patients do avail
themselves of that right. I do think you were
dealing with a patient who was perhaps a
little more educated or did a little more
homework than most.
DR LOVE: This case raises the question,
“When do we use a therapy that has not
been proven?” We know from our Patterns of
Care studies that in clinical practice, physicians
had not been using adjuvant trastuzumab
prior to the data becoming available,
and every time I interview a breast cancer
researcher they strongly discourage it.
Maybe this patient read Aman’s paper on
neoadjuvant trastuzumab and got excited
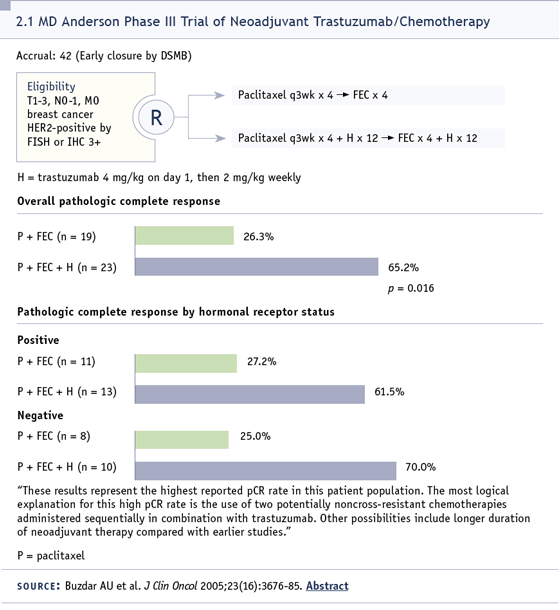
about the idea of adjuvant therapy (Buzdar
2005; [2.1]).
DR SMITH: She actually did read Aman’s
paper.
DR LOVE: Aman, why do you think physicians
were so hesitant to use adjuvant
trastuzumab off protocol?
DR BUZDAR: I think one of the major
concerns was that trastuzumab has a known
risk of cardiotoxicity and we didn’t know
the degree of benefit, so we didn’t know
whether the risk-benefit ratio would favor
treatment.
However, the results from our neoadjuvant
study showed substantial improvement in
pathological complete response, and I think
that, provided indirect evidence, the adjuvant
trials would be positive.
DR LOVE: It’s interesting that this educated
woman who was very aggressive about
wanting trastuzumab is content with tamoxifen,
which in a patient with three positive
nodes and HER2-positive disease, is not
aggressive hormone therapy. Has the issue
of ovarian suppression been raised in this
case?
DR SMITH: Yes, it’s been raised and she is
struggling with the thought of using leuprolide
or goserelin. I think what she’ll probably
do is have an oophorectomy.
If this patient opted not to do that, I’d
like to query what one would do with this
patient who is intramenopausal. She stopped
menstruating after the second or third cycle
of AC, but I don’t think she can be considered
permanently postmenopausal.
DR LOVE: This is a vexing and common question.
Aman, how do you approach endocrine
intervention in perimenopausal patients who
stop menstruating during chemotherapy,
particularly in the high-risk, HER2-positive
populations?
DR BUZDAR: There is no good way to define
these patients. The best thing we can do is
serial evaluations of the patient’s LH, FSH
and serum estradiol levels. If her LH and
FSH levels remain high and the serum estradiol
levels remain very low in the postmenopausal
range, chances are she will not
resume her cycles. Still, I think that if she does not want ovarian suppression, then it
is reasonable to start with tamoxifen.
DR LOCKER: I think we need to put this
all in context. We are discussing this issue
because women with ER-positive, PR-negative
disease and with HER overexpressed
tumors are two subsets of patients who,
based on several studies, don’t do as well
as the average patient on tamoxifen. Data
from ATAC and other trials and, in the case
of HER2-positive disease, data in the neoadjuvant
setting, demonstrate that these
patients do better on an aromatase inhibitor
(AI). So we’d like to see this patient on an
AI, but she can’t be on it unless she’s clearly
postmenopausal.
DR LOVE: Aman, what would you do if this
patient came to you requesting an LHRH
agonist and an aromatase inhibitor?
DR BUZDAR: The role of an LHRH agonist
with an aromatase inhibitor in premenopausal
women is under study, and a number
of protocols are ongoing. We know the
safety data. However, we don’t know the
efficacy of this regimen, and until we see
those data, I do not like to see it used in
clinical practice.
DR LOVE: What if the same patient came to
you requesting aromatase inhibitors after an
oophorectomy?
DR BUZDAR: Then there is no question that
I would put her on an aromatase inhibitor,
because she would then be postmenopausal.
DR LOVE: Aman, about half of the patients
in the adjuvant trastuzumab studies
presented at ASCO 2005 had ER-positive
tumors, although we don’t know the
quantitative levels. Did trastuzumab impact
these patients any differently?
DR BUZDAR: No. For these patients, appropriate
endocrine therapy should be offered,
because there is no question that endocrine
therapy can substantially change the natural
history of the disease in these patients, too.
DR LOVE: What do we know about combining
hormonal therapy and trastuzumab, in terms
of safety?
DR BUZDAR: In the NSABP study, patients
who received endocrine therapy received
trastuzumab concomitantly. No experimental
data exist to suggest any adverse interactions,
and none were reported.
DR LOVE: Gersh, it appears most physicians
are waiting until the patient completes
chemotherapy and then giving hormone
therapy along with trastuzumab, as they did
in the clinical trials.
DR LOCKER: Yes. In patients with HER2-
positive disease, recurrences occur early. In
the ATAC trial, an early blip was clear in time
to recurrence, even with hormone receptorpositive
disease. So you want to use your
best guns early — meaning hormonal
therapy and trastuzumab. I’d be very uncomfortable
waiting a year until trastuzumab is
completed.
DR LOVE: Let’s talk about chemotherapy in
patients with HER2-negative tumors and
positive nodes. How do you approach those
patients?
DR LOCKER: If the tumor were ER/PR-positive
and HER2-negative with multiple positive
nodes and the patient understood the
limited benefit of chemotherapy beyond the
benefit she gains from hormonal therapy,
I would use AC times four. I’m not even
convinced that adding paclitaxel makes a
difference. Now, if I were going to be more
aggressive for whatever reason — say she
has 20 nodes and wants to be as aggressive
as possible — then perhaps I would use TAC,
but that’s about as far as I’d go.
DR LOVE: What if the tumor were ER/PRnegative?
DR LOCKER: In a healthy patient with an
ER/PR-negative tumor and multiple positive
nodes, I would use TAC.
DR BUZDAR: TAC is a good combination,
but when you combine either docetaxel or
paclitaxel with other drugs, you increase the
morbidity. I think patients tolerate sequential
administration better, and you don’t
have to use growth factors to overcome
some of the side effects. My personal choice
would be to administer anthracycline-based
therapy followed by a taxane-based therapy
in these patients.
DR LOVE: How does the relative efficacy of
docetaxel compare with paclitaxel?
DR BUZDAR: In my judgment, the efficacy
of docetaxel every three weeks is similar
to weekly paclitaxel, whereas a study has
shown paclitaxel every three weeks is inferior
to docetaxel every three weeks in the
metastatic setting (Jones 2005).
DR LOVE: Gersh, do you think at some point
AC followed by nanoparticle albumin-bound
(nab) paclitaxel will be used?
DR LOCKER: It’s possible. Nab paclitaxel has
some advantages, but I’m not sure whether
the difference in efficacy, if there is any,
would warrant it. I think the bigger issue is
whether we’re going to be doing stratification
by receptors in terms of chemotherapy
choices, the way we do stratification by
receptors for hormonal therapy choices.
DR LOVE: Aman, if we find nab paclitaxel
is equal to paclitaxel in efficacy, will the
advantages of a shorter infusion time and
the ability to administer it without premedication
justify a shift in practice?
DR BUZDAR: Yes. I think the advantage of
nab paclitaxel is that you don’t have to use
steroids. When we use taxanes, one of the
major complaints from patients, besides the
neurotoxicity, is the weight gain and side
effects related to steroids. If you can avoid
that, you are actually enhancing the quality
of life of these patients. Even if the antitumor
activity of nab paclitaxel is identical
to paclitaxel, I think it has a better safety
profile.
Select publications
|
