You are here: Home: Meet The Professors Vol. 3 Issue 6 2005: Case 8

Edited excerpts from the discussion:
DR STEINEKER: This woman was 76 years old
and presented a year ago in July 2004 with
a Grade III DCIS in the left breast, for which
she had a modified radical mastectomy.
In February 2005, she was diagnosed with
an infiltrating ductal carcinoma, and a right
modified radical mastectomy was performed,
showing a Grade III ER/PR-negative, HER2-
positive tumor. The disease was staged as
T2/N0/M0, and her primary tumor was 3.5
centimeters.
I recommended the CALGB protocol 49909,
with chemotherapy and trastuzumab. She
was randomly assigned to Arm B, which was
AC followed by paclitaxel followed by trastuzumab.
That trial is a little bit of a challenge
because the investigators want to check
the FISH results, so the patient doesn’t find
out until they’re pretty far into the protocol
whether or not they’re going to receive the
trastuzumab.
She was treated with AC and had a lot of
trouble with that. I have not seen colitis
before, but she had diarrhea to the point
where we had to hospitalize her for a week.
She did have a preexisting history of diverticulosis
but did not have any particular
problem with that. According to the
protocol, we had to reduce her doses, and
she completed four cycles of AC. She then
started on paclitaxel. At the time I wrote
this up, she was into her ninth week, with
a fair amount of colitis and diarrhea despite
Imodium®. In fact, we had to hold the paclitaxel
for one week to let her recover. Her
blood counts were always good.
The dilemma is that, according to the
protocol, after the AC she needed a MUGA.
Compared to before the chemotherapy,
her MUGA had dropped from 66 to 50
percent, and the protocol had a cutoff of
15 percentage points. She was therefore
excluded from consideration for trastuzumab,
no matter what happened to the
ejection fractions.
Out of curiosity, though, I repeated her
MUGA one month after the AC and her MUGA
showed 71 percent left ventricular ejection
fraction, but to be careful, I did a 2-D
Echo, and this showed 45 percent. Anyway,
according to the protocol, she can’t receive
trastuzumab. She was at relatively low risk,
because she was lymph node-negative.
DR LOVE: Eric, what do we know about variations
in ejection fractions and 2-D Echos?
DR WINER: I don’t pretend to be an expert
in terms of the cardiac toxicity of these
agents. Clearly, measurements of cardiac
function can bounce around, and as Barbara
said earlier, getting MUGAs doesn’t prevent
heart failure, although it gives us some
clues about who may be at greater risk. I
would be concerned about giving her trastuzumab,
given the fact that in the study
the only data we have in terms of cardiac
toxicity are from patients whose ejection
fractions didn’t drop 15-plus percent.
Although you had that reassuring second
MUGA, you have an Echo that gives you a
very different result. So I think there is
reason to believe that her ejection fraction
has dropped with the anthracycline.
The issue of age is hard to deal with,
because on one hand, one doesn’t want
to discriminate against older women. On
the other hand, you want to include that
information appropriately in your decision-
making. Given the fact that events in
HER2-positive patients do tend to be earlier rather than later, it is likely that her life
expectancy will be such that she will not
die before she has a recurrence, if she’s
destined to have a recurrence. On the other
hand, this issue of toxicity is a big one, and
toxicity with one agent often correlates
with toxicity with another. I would just be
quite concerned.
I think the more interesting situation would
be if she were 45 years old or if she were 76
and had 10 positive lymph nodes. In those
situations, I would probably cautiously and
with a lot of discussion think about using
trastuzumab.
DR LOVE: Kevin, how are you approaching
the issue of trastuzumab for patients with
node-negative tumors?
DR FOX: NCCTG-N9831 did include node-negative
patients, although not many. My
feeling is that if they meet the criteria for
entry onto the study, they should be entitled
to the benefits of the therapy, if there
are no extenuating circumstances.
With respect to your patient, something
came to mind that I think everybody probably
has seen by now. When the NSABP did
their detailed cardiac analysis of the first
1,000 patients, this grid was produced that
has made the rounds, which perhaps you’ve
seen, which tries to identify the patients
who are at the highest risk for developing
trastuzumab-related cardiac problems. In
that grid, they positioned patients based
on their post-AC ejection fraction, and if
someone had fallen into the range of 50 to
54 percent, from another number, if they
had not fallen more than 15 points, they
were still eligible to go on, and they did.
If they fell to an ejection fraction range
between 50 and 54 percent and were
over the age of 50, in that small group of
patients, of which there were 47, the cardiac
event rate was 20 percent. That was the
subset that stood out as being uniquely
susceptible to the ill effects of trastuzumab.
Your patient is elderly and showed
a substantial drop in ejection fraction from
AC alone. That alone would raise a red flag,
that she has the potential, even under the
best of circumstances, for trouble later on.
So I, too, would be inherently reluctant to
use trastuzumab.
However, since we’re confessing things
today, I have had two younger patients
on clinical trials whose ejection fraction
dropped as yours did, unacceptably, and
were denied trastuzumab on the clinical
trial. These were people who, getting back
to the other case, were enrolled in 2003 and
now are presenting with normalized ejection
fractions and the same question that your
patient asked.
DR WINER: It would be a more interesting
dilemma if she had a number of positive
lymph nodes or if she were much younger.
In those situations, I think that I would
approach this situation a little bit less stringently.
In this case, I think that I’d fall back
on the guidelines in the trial and probably
not use trastuzumab.
DR STEINECKER: This lady had so much
trouble, she was so happy to hear she
wouldn’t be getting the trastuzumab once a
week for the next year that she was smiling
from ear to ear — for better or worse.
DR WINER: The CALGB has had this ongoing
trial that is slowly accruing older women,
comparing capecitabine with either CMF or
AC — “dealer’s choice.” Presumably, most
patients will get AC. This study is of women
over the age of 65 and now allows women
to receive trastuzumab after the completion
of chemotherapy. If I were seeing this
woman today, I might well encourage her to
enroll in the trial with the idea that after
the completion of chemotherapy, with the
knowledge of HERA, we would use trastuzumab
as a single agent at that point.
DR REEVES: I’m concerned about subclinical
heart disease with trastuzumab. Should we
be considering substituting epirubicin for
doxorubicin? Or should we be considering
dexrazoxane to protect people, if it’s going
to exclude our use of trastuzumab, which
we know now makes such a difference in
patients with HER2-positive disease?
DR WINER: This is a good question. The
results of the BCIRG trial imply that we’re
not ready to get rid of an anthracycline yet, and I think an important question is
whether we can make the anthracycline less
toxic. The CALGB had a study that enrolled
all of two patients about six years ago, evaluating
dexrazoxane and a variety of other
questions in the locally advanced setting
with trastuzumab. MD Anderson has been
using epirubicin concurrently with trastuzumab.
Other, less toxic anthracyclines, the
liposomal preparations, both Doxil® and
a drug that’s commonly called D-99, have
yet to be approved in the United States,
and may or may not ever be approved. I
believe it’s an important question and, of
course, if you could get the benefit from
an anthracycline with less concern about
cardiac toxicity, so much the better. Outside
of a trial, I wouldn’t be in a rush to give
dexrazoxane, but I think it’s an important
research question.
Everybody was hoping that the BCIRG study
would make this simple — that TCH would
be better, we wouldn’t be giving anthracyclines
any more and we’d be moving on to a
new era, in which preclinical assays would
predict how patients were going to do on a
routine basis. I don’t think we’re quite there.
DR LOVE: It never works out that easily,
does it?
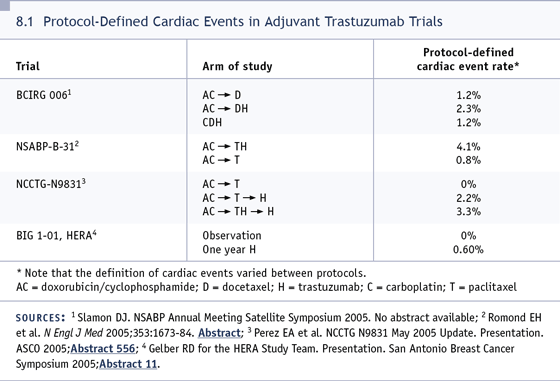
DR FOX: I don’t want this to come out
wrong, but how many people were really put
in harm’s way, clinically, by the cardiotoxicity
(8.1)? I’m not diminishing the fact that
there were a couple of cardiac deaths. In
the HERA trial, the rate of congestive heart
failure for those who received sequential
therapy was remarkably low, and maybe we
ought not allow this to be more of a concern
than it merits. This was a large clinical trial
where far fewer people came into harm’s
way from cardiac toxicity than they did from
recurrence and death from breast cancer had
they not received trastuzumab.
With stringent monitoring, if we all just
adhere consistently to the protocol criteria
and we pay close attention to this post-AC
ejection fraction, which appears maybe to
be quite important, I don’t think we’ll have
too much trouble, and we won’t need to use
more expensive therapies like epirubicin and
dexrazoxane to offset the problem.
DR LOVE: Eric, what kind of clinical scenario
in terms of risk for cardiovascular disease
would have you considering TCH right now,
and how much of a clinical history would
make you say, even in a node-positive
patient, “I’m not going to use trastuzumab”?
DR WINER: As long as a patient had an
acceptable ejection fraction level, there
isn’t any cardiac risk factor that would push
me in the direction of not using trastuzumab
in that setting.
I think where this becomes an issue is with
a patient with a 1.3-centimeter ER/PR-negative,
node-negative tumor, who was just
barely eligible both for HERA and for the
Intergroup trial, or the patient who’s got a
slightly larger ER-positive tumor, who might
have been eligible for HERA but wasn’t
eligible for the Intergroup trial. In those
situations, there’s good reason to think that
the relative risk reduction with trastuzumab
will be the same across the board, but it’s
not relative risk reduction that should push
us to decide to give a therapy. It’s the absolute
reduction. That’s where you’ve got to
really think about the toxicity issues.
DR LOVE: So what about a patient with
normal ejection fraction but a history of a
couple of MIs and hypertension?
DR WINER: In the setting of a normal ejection
fraction in somebody who’s got a relatively
high risk of recurrence — node-positive
disease — I would probably use trastuzumab
in those patients who were eligible
for the trial.
DR LOVE: Which chemotherapy?
DR WINER: Would I give TCH or AC followed
by paclitaxel? The problem is that I think
TCH is a pretty toxic regimen, and I’m not
sure in which patients I would use it.
I suppose I would use it in a younger woman
who I thought could tolerate the other
toxicities, other than the cardiac issues,
who either has a borderline ejection fraction
— and occasionally, you’ll come across
somebody, a 38- or 42-year-old woman, who
has an ejection fraction of 48 percent — or
someone, perhaps, who’s had Hodgkin’s
disease before and had mantle irradiation,
and I’m more concerned about cardiac
issues. At the moment, I’m not quite sure for
whom I would use it.
DR FOX: With these questions in mind,
there was actually a case that’s come up
just in the last week. I treated a patient in
1989 for a T2/N1, ER-negative right breast
cancer. She received six cycles of CAF, 360
mg/m2 of doxorubicin and has been fine.
She has a normal ejection fraction currently
but has a contralateral breast cancer that
is 2.5 centimeters, ER-negative, HER2-positive,
node-negative. I’m not going to give
her anthracyclines. Would I administer TCH?
It looks like maybe it’s my default position,
as much as my enthusiasm for doing it has
gone down.
DR WINER: I think Kevin’s case is perhaps
the best of all of them in terms of the situation
in which I would administer TCH, which
is for someone who can’t receive an anthracycline.
It’s not even an issue. You’re not
going to give it to this woman who’s had
360 mg/m2 of doxorubicin. At the moment,
this is someone to whom I absolutely would
give TCH.
DR FOX: Now, had I treated her two years
later, when the patterns of care were
changing a bit, I probably would have given
her AC, and then it would be 240 mg/m2.
Would that make you feel any differently?
DR WINER: I think I’d still give her TCH
today.
DR LOVE: Would you have given her TCH
before the press release came out from the
BCIRG?
DR WINER: I probably would have because,
at a minimum, you can’t give her an anthracycline,
and it’s a regimen that we know
has been given to a large group of women,
and so there’s at least a toxicity experience
that’s been amassed.
DR LOVE: Would you be surprised if it turns
out that TCH is not as effective as the
anthracycline-containing regimen?
DR WINER: Right now, we don’t know that
it isn’t as effective. But if it turns out to be
the case, I think the real question will be
which patients need an anthracycline in the
HER2-positive setting and which don’t.
It doesn’t surprise me that a subgroup
of patients exists who benefit from an
anthracycline, given all of the data that
suggest that the benefits of anthracycline-
based regimens, compared to CMF, are largely confined to women with HER2-positive
disease, albeit retrospective — but
convincing — analyses.
We need to spend time focusing on molecular
predictors of resistance to trastuzumab.
As a medical community and a breast cancer
research community, it’s almost shameful
that we haven’t figured this out, in spite of
the fact that we’ve been using trastuzumab
in the metastatic setting for seven or eight
years.
Much of the problem relates to the fact
that it just hasn’t been in our practice
approach to perform biopsies at the time
of relapse. If we had 100 patients who
had been on trastuzumab and experienced
disease progression, had biopsies, and we
had interrogated the tissue, we might have
some clues. But we do have clues based on
preclinical data.
DR LOVE: The C-Myc data done by
Soonmyung Paik was able to separate
out a group that had a greater than 90
percent chance of remaining disease free,
as opposed to a group whose chance of
remaining disease free was in the 60s. It
reminds me of some of the work he’s done
with Genomic Health, which has some teeth
to it in terms of decision-making. Maybe
we’ll have trials that’ll focus on the people
who we think have high relapse rates.
DR WINER: Right, and those are the
patients for whom you might consider strategies
such as other HER2-directed therapies
in place of trastuzumab or therapies in addition
to trastuzumab.
DR LOVE: Kevin, what were your thoughts
about Edith Perez’s NCCTG data suggesting
an advantage to concurrent versus sequential
chemo-trastuzumab?
DR FOX: She did, indeed, draw that conclusion,
but I think she was quick to point out
that at that point in time, the number of
recurrences was very small. So it’s too early
to conclude that we know the worth or lack
of worth of sequential versus concurrent
treatment. We just don’t know.
DR LOVE: There is a lot of confusion about
this exact point, because the HERA study,
which is seemingly a similar sequential
strategy, showed a 50 percent reduction in
relapse rate.
DR WINER: I think the HERA results are
impressive and stand on their own without
a lot of difficulty. It is quite possible that
concurrent may be better than sequential,
but we don’t know that at the moment. The
only reason we know anything from N9831
about sequential versus concurrent therapy
is that when the DSMV met and decided to
release the data about trastuzumab, as a
practice management question in terms of
what to tell doctors whose patients were
on the trial, they asked to look at those
two arms, so that they could give doctors a
sense of what to do for those patients who
had been treated on the trial and didn’t
receive trastuzumab.
While there is a statistically significant
difference between the concurrent and
sequential arms on Edith’s trial, and the
sequential arm wasn’t significantly better
than no trastuzumab, it did not meet any
boundary in terms of early stopping. I think
that we just need more data.
DR STEINECKER: So if Edith’s study shows
no benefit for that sequence, then the
timing of one year, two years, is shot.
DR WINER: We know there’s benefit from
HERA. The risk reduction in HERA was
similar to what we’ve seen in all of the
studies. All of these studies — other than
that one arm in N9831 — have shown
that the use of trastuzumab either with or
following chemotherapy reduces the risk of
disease recurrence by about half, and the
results are shockingly consistent.
DR LOVE: At the NSABP meeting, people
were talking about the fact that most of
the HERA patients did not receive taxanes.
Could that be confounding this? If you’re
going to give the patient a taxane, isn’t it
going to bump up the efficacy and, in some
way, dampen what you’d see with trastuzumab?
DR WINER: Perhaps. What has been stated
incorrectly is that most of the patients in
HERA didn’t receive an anthracycline. In fact, 92 or 94 percent of patients in HERA
received an anthracycline, and I think about
a third of them received a taxane.
It’s very difficult to compare across trials. I
think there’s a signal from Edith’s trial. I’m
not saying it should be ignored, but I would
not conclude, based on her data, particularly
considering HERA, that trastuzumab after
chemotherapy is ineffective. That would
be an inappropriate conclusion. I think an
appropriate conclusion is that — particularly
if you’re giving a taxane — sequential
therapy isn’t as good as concurrent, but we
have to see.
DR LOVE: The schedule of how the
paclitaxel was administered originally was
weekly, and then every three weeks. Does
that make a difference?
DR WINER: In N9831, it was administered
as a weekly regimen. In the NSABP trial, it
was initially administered every three weeks,
and then the study was amended and it was
allowed to be administered weekly. I don’t
know whether it matters or not. Certainly
the suggestion exists that weekly paclitaxel
is better than every three-week paclitaxel.
Whether that matters in the context
of HER2-positive disease and with concurrent
or sequential trastuzumab, we don’t
know. If I were going to use it, I would tend
to use the weekly schedule as was done in
the studies.
Select publications
|
