You are here: Home: Meet The Professors Vol. 3 Issue 6 2005: Case 3

Edited excerpts from the discussion:
DR MARCOM: This patient is a 45-year-old
woman, who presented first in May 1999
with bilateral breast cancer. She had a right
breast tumor that was a T2 lesion, about 2.4
centimeters, Grade II, ER 10 percent, PR 10
percent, HER2 1+. She also had a contralateral
breast tumor that was 1.2 centimeters
and was also node-negative, ER and PR also
10 percent and HER2-negative.
She underwent bilateral mastectomies,
very much at her preference, and received
four cycles of AC. She was premenopausal
and was placed on tamoxifen for borderline
hormone receptor-positive breast cancer.
She did well and stayed on tamoxifen for
five full years, but then presented in May
2005 to her internist with severe right upper
quadrant abdominal pain. She had the clinical
appearances of metastatic disease that,
in my opinion, was bordering on visceral
crisis.
She had essentially complete replacement of
her left hepatic lobe and significant disease
in her right hepatic lobe also. Her baseline
alkaline phosphatase was 205, SGOT was 166
and SGPT was 61, but she was in quite a bit
of discomfort. She also had some retroperitoneal
mediastinal lymph nodes.
We biopsied the liver lesions, which were ERand
PR-negative and HER2-negative — 1+
by IHC and FISH negative. A PET scan also
revealed a pericardiac mass that was not
hemodynamically compromising, retroperitoneal
nodes and a right adnexal mass.
DR LOVE: Can you talk about her lifestyle
and family situation?
DR MARCOM: She’s a “salt-of-the-earth”
person. She’s a stay-at-home mother. Her
husband has a blue-collar job, and her children
are nine and 12 years old. She’s just a
very solid woman, one of these tragic cases
that we all see in young women with aggressive
metastatic breast cancer in the midst of
trying to raise their families.
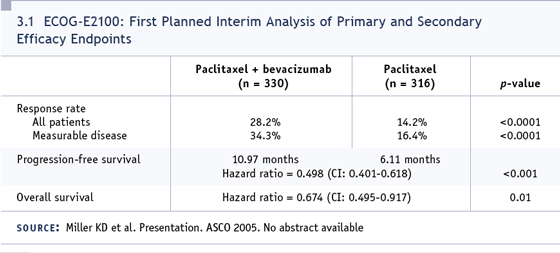
DR LOVE: Therapy was initiated on May
26th of 2005, which was just 10 days after
the bevacizumab presentation at the ASCO
meeting (Miller 2005a; [3.1]). With that
in mind, Gersh, how would you treat this
unusual case of a young patient in visceral
crisis with metastatic disease?
DR LOCKER: It’s a good point, because in
the average patient with metastatic disease
that’s ER-negative, there is little advantage
to using anything other than sequential
single-agent chemotherapy. The only
advantage to using combinations is a
higher response rate and probably a quicker
response rate. So in this case, a taxane is a
given — it’s the most active drug that she
hasn’t already received — and this would be
a patient for whom I would add something
to a taxane. One key issue is her liver function.
In a patient such as this, the liver enzymes
are a good reason to use a low-dose weekly
paclitaxel regimen, because you’re going
to get into less trouble than giving either
docetaxel or paclitaxel every three weeks.
Now, what would I add to low-dose weekly
paclitaxel? Data were presented at ASCO in
favor of adding bevacizumab and that’s one
alternative (Miller 2005a). The other would be to add something like a fluoropyrimidine,
since there’s data on capecitabine (Susnjar
2005; Uhlmann 2004).
To be honest, I’m not certain what I would
do. Even though I was there for the presentation
of the bevacizumab data, I’d probably
add a second chemotherapeutic
agent, understanding it probably will not
impact survival. It’s just going to allow
you to treat her quicker and get a greater
response. Fluoropyrimidines are attractive
because they don’t need liver metabolism.
Doxorubicin is perfectly reasonable to
combine with docetaxel, but not in a patient
with liver disease.
DR LOVE: Would you treat her any differently
if she had not received any prior
chemotherapy?
DR LOCKER: Again, the most active regimen
probably is a combination of a taxane and
doxorubicin, specifically docetaxel/doxorubicin
in this case, but my big concern is the
liver function. You are going to have problems
with stomatitis and GI toxicity if you
do that, even if you administer cytokines. I
think I would use low-dose weekly paclitaxel
and 5-FU or capecitabine, and less likely
bevacizumab.
DR BUZDAR: For this lady I would discuss
various options, but I would recommend
combination chemotherapy. The
other option I would discuss with this
patient is paclitaxel with bevacizumab.
This patient has extensive replacement of
the liver and I think it would be appropriate
to utilize combination therapy to get
a quick response, such as capecitabine or
gemcitabine with a taxane.
DR LOVE: What about combination chemotherapy
and bevacizumab?
DR BUZDAR: We don’t have any safety or
efficacy data on the combination of bevacizumab
with two drugs. If we had even Phase
I data indicating that you could combine the
drugs, I would have gone with that (Miller
2005a, 2005b).
DR LOVE: Dr Marcom, what happened with
this patient?
DR MARCOM: I would generally use singleagent
therapy and I don’t think of myself
as somebody who pushes the envelope too
much, but I had just returned from ASCO
and seen the bevacizumab data. In my
opinion, this case was a kind of the basallike
breast cancer subtype. I fully discounted
her original ER and PR and saw her case as
aggressive and I felt she needed combination
chemotherapy.
With all that said, I gave her weekly paclitaxel
along with capecitabine and bevacizumab.
She actually has had quite a
remarkable response and has tolerated
therapy extremely well (3.2). I was part of
Bill Gradishar’s capecitabine plus paclitaxel
study and was impressed that it was
a pretty well-tolerated regimen (Gradishar
2004; [3.3]), and we have safety data on the
combination of capecitabine and bevacizumab
in breast cancer (Miller 2005b), so I
felt I had a leg to stand on.
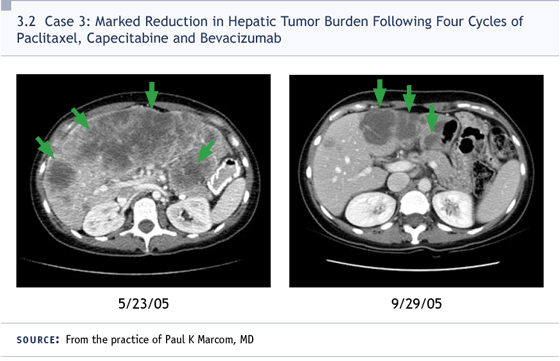
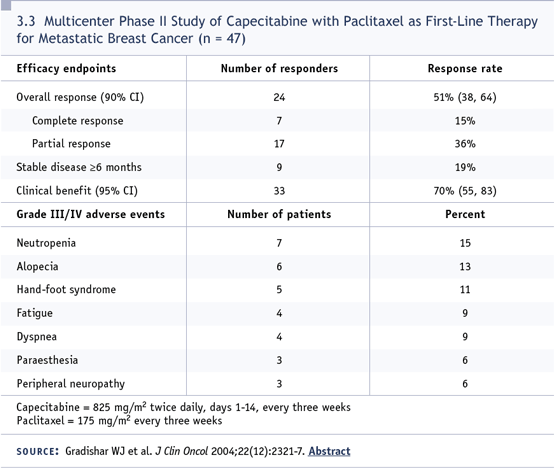
DR LOVE: How many courses has she
received?
DR MARCOM: She’s received four cycles altogether
now. She had very quick symptomatic
improvement about halfway through the second cycle. Her right upper quadrant pain
decreased quite dramatically. My plan was
that I would drop the capecitabine at some
point once I had gotten a response.
DR LOVE: Have you considered stopping
the paclitaxel or even both chemotherapy
agents?
DR MARCOM: That’s a very good question.
Looking at ECOG data on paclitaxel plus
bevacizumab, I think I have the best justification
for stopping the capecitabine, at
least initially. I would be hesitant to stop all
chemotherapy and have her on just singleagent
bevacizumab, given the response that
we have for that (Cobleigh 2003).
DR LOVE: Aman, if this patient came to you
for a second opinion a few months from now
and she’d had a great response, and she were
stable, what would you recommend at that
point?
DR BUZDAR: Our approach is that if she
were not experiencing excessive toxicity, we
would advise her to continue the therapy,
unless she had a complete clinical response.
DR MARCOM: For what it’s worth, her CA15-3
started at 331 U/mL and has normalized
now. Hopefully, her scans will fully catch up
with that if this is necrotic tumor left in her
left lobe.
DR LOVE: In talking to Bill Gradishar about
the capecitabine/paclitaxel study and
Joanne Blum from US Oncology who looked
at a similar regimen, it seemed they felt
the combination was not necessarily more
effective than other combinations — for
example, docetaxel combinations — but
that it was better tolerated. What was your
impression?
DR MARCOM: It’s been my general sense
that that is the case.
DR LOVE: Aman, you’re evaluating
capecitabine with docetaxel in your neoadjuvant/
adjuvant trial. Can you talk about
that study and what you’ve observed in
terms of tolerance and the dose?
DR BUZDAR: We started that study a couple
of years ago with 1,000 mg/m2 twice daily
of capecitabine, but after we treated
a few patients, we had to modify the
dose due to toxicities. The current dose
of capecitabine is 750 mg/m2 twice a day
for two of three weeks with docetaxel 75
mg/m2 every three weeks. That regimen
is fairly well tolerated and we have now
treated a sizable number of patients. Also,
this regimen can be given on an outpatient
basis. I don’t think 1,250 mg/m2, the
package insert dose of capecitabine, can be
tolerated by most patients (3.4).
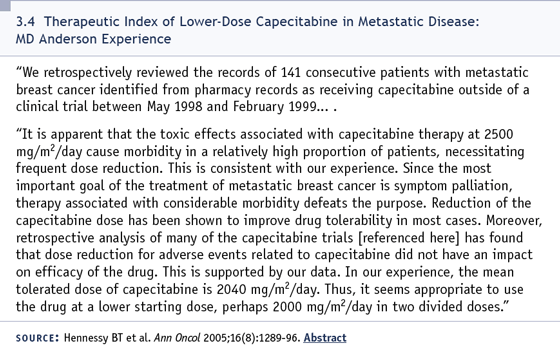
Select publications
|
