You are here: Home: Meet The Professors Vol. 3 Issue 6 2005: Case 6

Edited excerpts from the discussion:
DR DRAGON: This 55-year-old woman
presented with a locally advanced, ER-positive,
PR-positive, left breast tumor and bone
metastases approximately three years ago.
She underwent a subtotal mastectomy, axillary
dissection and reconstruction. She was
given tamoxifen, to which she responded,
and then letrozole, which also resulted in
significant disease control.
DR LOVE: Did this patient know she had
breast cancer and neglect it or was she just
one of those cases that presents with metastatic
disease?
DR DRAGON: She was very frightened. She
had been treated for DCIS a number of years
earlier with a right mastectomy and then
had reconstruction. She knew there was
something wrong in her left breast and she
did not want to deal with having another
episode of breast cancer.
DR LOVE: Was the left breast tumor an
obvious lesion?
DR DRAGON: At the time I saw her, she had
a four-centimeter mass that was fixed to the
skin and clearly obvious as a breast cancer,
and she knew that.
DR LOVE: Did she have bone pain from the
metastases?
DR DRAGON: No.
DR LOVE: What was her life situation at that
time?
DR DRAGON: She is a fashion model in her
second marriage with two kids. Her son is a
medical resident at a university center. She
travels internationally on a regular basis.
Her husband is a businessman in the community.
They are independent financially and
very secure.
DR LOVE: When she presented with locally
advanced disease, how would you describe
her mental state?
DR DRAGON: That’s an interesting question.
When she first presented to the breast
surgeon and he sat down with her to explain
that this looked like breast cancer, she
became very unstable emotionally and actually
had to be hospitalized for a couple of
days.
She went directly from the surgeon’s
office to an inpatient psychiatric unit. She
improved very rapidly, then came to see
me and was ready to hear about how to get
better.
DR LOVE: What was her psychological and
medical history?
DR DRAGON: She had never had any medical
or psychiatric illnesses before, except for
the DCIS.
DR LOVE: How did she react to the idea of
hormonal therapy?
DR DRAGON: Hormonal therapy fit in very
well with her lifestyle. She was able to
maintain an active travel schedule, seeing
me between her travels to Europe, the Far
East and Hawaii. This is a woman who was
used to traveling a great deal and continued
to do so while on therapy.
DR LOVE: How did she do emotionally after
her initial reaction?
DR DRAGON: During periods when her
disease was clearly beginning to progress,
she would become tearful, but with a
little bit of support and emphasis about the
potential control of her disease with other
therapies, she readily compensated within
the span of an office visit and was able to
go back to normal functioning.
DR LOVE: What hormonal therapy did she
receive?
DR DRAGON: We started with tamoxifen
and she achieved excellent disease control.
When we reached an optimal level of control
in the breast, the surgeon did a wide excision
and cleaned out her axilla for local
control, because initially this was an ulcerated
lesion. We did not radiate the local
area. She had excellent control of her bone
metastases as well.
DR LOVE: What prompted the decision to
use tamoxifen initially as opposed to an
aromatase inhibitor?
DR DRAGON: It’s a good question. We began
therapy five years ago. If I saw this patient
today, I would probably start with an aromatase
inhibitor.
DR LOVE: After tamoxifen she received
letrozole. How did she tolerate endocrine
therapy?
DR DRAGON: She did not experience any
toxicity with either agent. When she
progressed, it was primarily in the breast.
She never developed more bone metastases
or pain.
We then started fulvestrant, which
was approximately 18 months ago. She
received fulvestrant for four months, and
we did not give her a loading dose. She
clearly progressed on the chest wall and
in the axilla and at that point, we began
discussing systemic chemotherapy.
DR LOVE: Eric, what options would you have
presented to this patient at this point?
DR WINER: I wouldn’t have totally ruled out
using another hormone, but it’s certainly
reasonable to move on to chemotherapy at
this point.
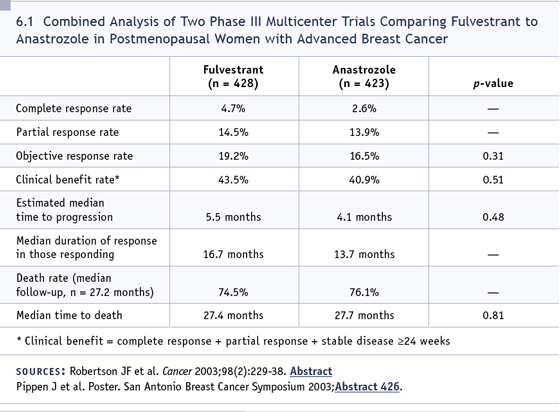
I would like to comment on fulvestrant. It’s
perplexing to a lot of people that it doesn’t
perform better given that in the randomized
trials it was at least as good as, if not
a little bit better than anastrozole (6.1).
Some of that may relate to the setting.
There’s some concern that using it after an
aromatase inhibitor may not be the optimal
setting for this agent, although it’s the way
we all give it. Ongoing studies are evaluating
estrogen priming for brief periods of
time after an aromatase inhibitor followed
by fulvestrant. Then there’s the issue of a
loading dose. It may turn out that one of
the problems is that it just takes a long time
to get to an optimal therapeutic level.
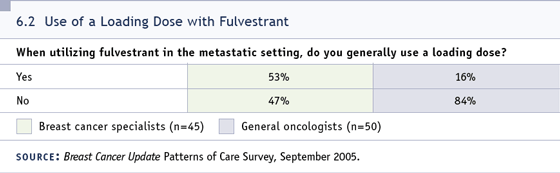
DR LOVE: Do you use a loading dose?
DR WINER: I haven’t outside of a study
(6.2). I don’t think we know yet the optimal
way to administer fulvestrant, and there’s a
lot of interest in learning the most effective
way to use it. One reason why there have
been delays in initiating any type of large
adjuvant trial with fulvestrant is that until
we define the optimal way to use this drug,
we’re just shooting ourselves in the foot if
we try to start a trial sooner.
DR LOVE: If you were to begin chemotherapy
at this time, what regimen would
you choose?
DR WINER: As a general rule, I’m a singleagent
guy and I’m not convinced that combination
therapy is superior to giving single
agents sequentially. The two trials that have
shown that combination therapy may be
superior — that is, the docetaxel with or
without capecitabine study and the paclitaxel
with or without gemcitabine trial
— are both flawed in that there was not
an appropriate crossover (O’Shaughnessy
2002; Albain 2004). In the studies that have
evaluated crossover, there is absolutely no
difference in survival between single agents
and combination therapy (Sledge 2003).
So, in this woman who is not terribly symptomatic
and for whom you want to do your
best to minimize the impact of therapy on
her quality of life, be as specific as you can
with the therapy and eliminate drugs that
aren’t working, I would definitely use single-agent
chemotherapy. In my view, it almost
doesn’t matter whether you use capecitabine
or a taxane or an anthracycline. For that
matter, you could probably use gemcitabine
or vinorelbine, although they’re less
commonly used in this situation. I believe
that response rates and time to progression
are more dependent on when you administer
the drug than on which drug it is.
DR LOVE: Would you consider incorporating
bevacizumab?
DR WINER: This patient would have been
eligible for the ECOG trial with bevacizumab.
The results presented at ASCO showed
approximately a five-month improvement in
time to progression and a doubling of the
response rate (Miller 2005a). Unfortunately,
bevacizumab is a very expensive drug, for
which we don’t have an identified target.
We just don’t know who benefits and who
doesn’t, or perhaps everybody benefits a
little bit.
If I were going to use bevacizumab, I would
give bevacizumab in combination with paclitaxel,
the way it was done in the ECOG trial.
DR LOVE: Dr Dragon, what happened with
this patient?
DR DRAGON: This predated the bevacizumab
data, which I still don’t know what
to do with. We talked about the options and
had a similar conversation about the many
different choices of sequential single agents
and agreed that ultimately she would probably
see a lot of different drugs. We offered
capecitabine as a reasonable option that
would allow her to maintain her lifestyle,
and she found an oral agent to be very
appealing.
DR LOVE: How important was the issue of
alopecia for her?
DR DRAGON: At this point, very important.
She understands that, at some point,
that’s going to be an issue, but the availability
of drugs — including vinorelbine,
gemcitabine and capecitabine — that don’t
cause significant alopecia basically allowed
her to compensate for the loss of control in
her life.
DR LOVE: Eric, if you had seen this
woman before the bevacizumab data were
presented, what therapy would you have
used?
DR WINER: Capecitabine.
DR LOVE: Would that change now that we
have the bevacizumab data?
DR WINER: Since those data became available,
I tend to somewhat cautiously use
bevacizumab in this setting, and when I do,
I’m a little uncomfortable combining it with
capecitabine, given the negative data from
Kathy Miller’s previous study (Miller 2005b).
Now, I don’t know if that trial was negative
because bevacizumab isn’t as effective in
combination with capecitabine as it is with
paclitaxel or whether it’s because of the
setting — that is, first line versus not first
line. I tend to think it’s probably not agent
specific but rather more related to the fact
that the ECOG paclitaxel trial was conducted
with patients who had not received prior
treatment in the metastatic setting.
I think that even today, I probably would
pick capecitabine alone and hold off on
doing anything else in this particular
patient.
DR LOVE: Kevin, how would you think
through this decision?
DR FOX: The same way. The decision would
rest entirely upon which drug fits the
patient’s lifestyle, desires and limitations.
This patient seems like a logical and almost
perfect candidate for capecitabine.
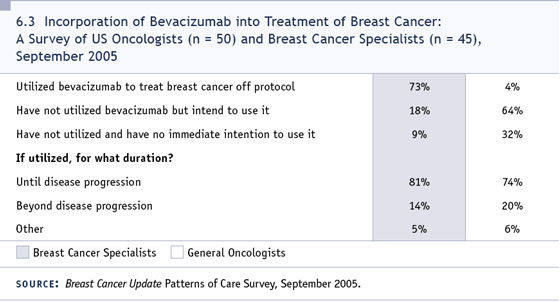
DR LOVE: Eric, I think a lot of physicians
share Dr Dragon’s concern about not knowing
how to apply the bevacizumab data (6.3).
How would you respond to this concern?
DR WINER: I think part of the reason that
people are uncertain is that we don’t have
a huge amount of data on bevacizumab. We
have these two studies and we don’t know
much about bevacizumab with other agents.
We’ll probably learn more in the next few
years.
My other comment is that this is a woman
who has never received any chemotherapy
before, so even if she receives capecitabine
initially and you plan to use bevacizumab
in the second-line setting, she’s still more
like the ECOG patients, and so I don’t think
you’re burning any bridges by giving her
single-agent capecitabine.
DR LOVE: Kevin, what do you think about
using bevacizumab in the second-line
setting in a patient who has received no
prior adjuvant therapy?
DR FOX: I think it makes perfectly logical
sense. I don’t think we ought to make
blanket rules about the use of bevacizumab
exclusively as first-line therapy, because not
every patient will be like the patients who
were actually in the clinical trial.
DR LOVE: Dr Dragon, can you follow up on
what happened with this woman?
DR DRAGON: She was started on
capecitabine 10 months ago and had a very
rapid and gratifying response. The node
went away and the skin disease disappeared.
Her bone disease was already asymptomatic.
The response continued for more than nine
months, and just this last month, her node
became palpable. Her skin disease has still
not recurred, but I think she’s starting to
progress.
DR LOVE: Eric, what are your thoughts about
the dosing of capecitabine?
DR WINER: I usually don’t bother calculating
a precise dose per meter squared. I
never use the 150-milligram pills. I’d start
a normal-sized woman at 1,500 milligrams
twice a day, and then adjust as necessary.
Clearly, responses are seen at those doses.
I know that Larry Norton has been talking
about some interesting scheduling ideas on
capecitabine dosing. It may be a drug that
will be more effective with different dosing,
but at the moment, I think we’re left dosing
it with a modification of the package insert, meaning two weeks on, one week off, but
with lower doses.
DR LOVE: What about other taxanes,
docetaxel and nab paclitaxel, in combination
with bevacizumab?
DR WINER: I tend to be somebody who likes
to see a little data. I don’t see a reason for
giving nab paclitaxel with bevacizumab at
the moment. Once there are some safety
data with the combination, and I don’t have
any reason to think that it won’t be safe,
then I think that’s fine.
DR FOX: Eric, do you foresee using single agent
bevacizumab under any circumstances?
DR WINER: Well, in the study that Melody
Cobleigh, George Sledge and Kathy Miller did
evaluating bevacizumab as a single agent, it
showed a bit of single-agent activity, mostly
in heavily pretreated patients (Cobleigh
2003). I probably wouldn’t be in a rush to do
that at the moment, but I think it’s a question
to be asked in clinical trials.
One other thing I want to mention about the
ECOG trial is that unlike most of the previous
studies we have evaluating paclitaxel or any
agent, the ECOG trial is one of the first that
actually systematically excluded patients
with HER2-positive disease. As we winnow
down the patient populations, there may be
somewhat unexpected findings in terms of
response rate and time to progression. So in
that ECOG trial, two-thirds of the patients
had ER-positive disease, like this patient,
and a third of the patients had ER-negative
disease, which in that study, by definition,
was triple-negative disease.
DR DESAI: Eric, do you feel the ECOG study
data should change the way we practice?
DR WINER: I think that we’re not quite
sure what to do with the data. In my talk
at ASCO, I carefully chose my words and
said that it was reasonable to use bevacizumab
in settings similar to the ECOG trial.
However, it’s not mandatory, and different
people will approach this question in
different ways. Hopefully we’ll have some
more data in the not-distant future.
DR LOVE: It’s been interesting to see how
in breast cancer the reaction to the bevacizumab
data has been completely different
from what we saw in colon and lung cancer.
In colon cancer, people jumped on the data
and actually started using it with FOLFOX,
even though the trial was with IFL. In lung
cancer, reimbursement issues exist, but the
researchers feel that once it is reimbursable,
they’ll begin using it.
DR FOX: I think this stems from the fact
that in the treatment of metastatic breast
cancer we have lots of choices, whereas up
until recently there were few choices, and
even fewer good choices, in treating lung
and colon cancer. As a result, we tend to be a little bit more circumspect with respect to
what we’re going to use and what kinds of
toxicities we’re willing to accept.
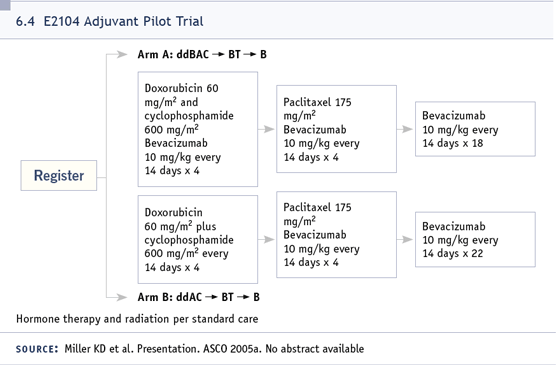
DR LOVE: Eric, when do you think we’ll
begin to see adjuvant bevacizumab
breast trials?
DR WINER: In the not-too-distant future.
ECOG will be sponsoring a pilot study similar
to the pilot feasibility study we saw with
trastuzumab many years ago. A Phase III
concept has already been submitted to the
NCI from ECOG, so I suspect that study will
open in 12 to 24 months, and I think it’s a
reasonable study (6.4).
Select publications
|
