You are here: Home: Meet The Professors Vol. 3 Issue 6 2005: Case 4

Edited excerpts from the discussion:
DR WEINER: This 38-year-old woman
presented in January 2004 with a 1.5-centimeter,
poorly differentiated, ER-positive,
PR-negative, HER2-negative, intraductal
breast cancer. Vascular invasion was noted,
and four lymph nodes were positive.
She was initially treated with dose-dense
AC followed by paclitaxel and radiation
therapy to the left breast and axilla. She
was prescribed tamoxifen, 20 milligrams
daily. Her last menstrual period was at the
onset of chemotherapy.
DR LOVE: Kevin, this patient’s last
menstrual period was less than a year ago
and she’s on tamoxifen. Would it be appropriate
to switch her to an aromatase inhibitor?
DR FOX: I think it would be appropriate
to switch a patient from tamoxifen to
an aromatase inhibitor after two years of
therapy. However, that requires that the
patient be in true menopause, which brings
up a very important issue in this case: When
can you be assured that this patient is truly
in menopause? I think it’s safe to say that,
for the first time this year, we were given
some information that gives us a clue as to
the natural history of chemotherapy-induced
amenorrhea and its permanence, or lack of
permanence, in women. I had never seen
much on this issue before.
The late Dr Jeanne Petrek from Memorial was
one of the organizers of a multi-institutional
study, wherein newly diagnosed patients
were recruited during or shortly after they
completed adjuvant therapy. All that the
study required was menstrual histories from
these patients, essentially on a daily basis,
for a three-year period. The data presented
at ASCO (Petrek 2005) gave us an idea about
how often women became amenorrheic and
how often their menstrual periods resumed.
Without belaboring the details, the most
compelling observation was that if you
looked at the percentage of patients after
chemotherapy who were in a state of amenorrhea,
the data were convincing that no
reversibility remained after the second year.
On the other hand, quite a bit of reversibility
was evident after the first year, especially
in women under the age of 40. The
point being that the premature prescription
of an aromatase inhibitor might result in a
therapeutic failure if the patient still has
ovarian function.
What we’ve done, as an unofficial policy,
is that if we need to confirm the patient’s
menstrual status, we check their estradiol
and FSH levels, for all its faults. We haven’t
been burned thus far.
DR LOVE: Eric, this is a 38-year-old woman
with four positive nodes. We know that
in the postmenopausal woman, aromatase
inhibitors reduce recurrence risk more than
tamoxifen. What are your thoughts on how
best to treat this perimenopausal patient?
DR WINER: The truth is, in a woman who is
premenopausal at diagnosis, we don’t know
that any aromatase inhibitor used in the
first five years is better than tamoxifen. No
such patients were included in the trials,
other than the MA17 trial, which involved
treatment after five years of tamoxifen. If
you think about it, a premenopausal woman
experiences ovarian suppression from
chemotherapy, and if she is on tamoxifen
she has received essentially two hormonal
therapies, one of which is substantially lowering her estrogen levels.
It’s an important and unanswered question
as to whether, in a premenopausal woman,
ovarian suppression and an aromatase inhibitor
are better than ovarian suppression with
tamoxifen. That is the question being asked
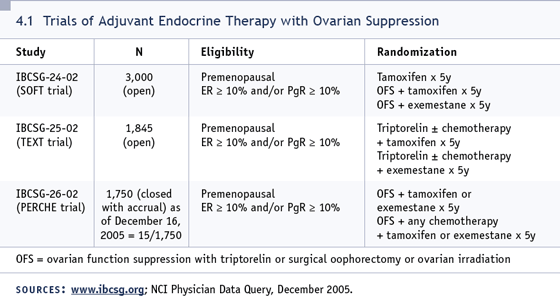
in the TEXT and SOFT trials, and I could
imagine the results going either way —
showing the aromatase inhibitor combination
to be superior or inferior to the tamoxifen
combination (4.1).
DR LOVE: Do you think that ovarian
ablation and tamoxifen will be better
than tamoxifen?
DR WINER: I suspect that it may be, but
that wouldn’t keep me from enrolling
someone in the study, because I’m not
sufficiently convinced. The decision to add
ovarian suppression and tamoxifen to treat
a woman who’s still premenopausal after
chemotherapy is a tough decision outside
of a trial.
I do it occasionally, and sufficient data
exist to make me comfortable with that,
but at the same time, I wouldn’t say it’s the
standard. However, I would be worried that
this woman might start cycling again if you
switch her too soon, and we don’t know that
switching at any point in time will improve
her outcome.
DR LOVE: If she came to see you in another
couple of years and had now not menstruated
for three years, would you switch her
then?
DR WINER: I’m still a little nervous in a
38-year-old woman. If she were 48, had
received AC followed by T and had not
menstruated for three years, I’d be pretty
comfortable. Although I realize those
patients weren’t included in IES or the
ABCSG ARNO study, I tend to switch those
patients (Coombes 2004; Jakesz 2005). On
the other hand, if this 38-year-old patient
were menopausal at the four- to five-year
point, I would switch to an AI then or
perhaps sooner if we have additional data
before then.
DR LOVE: Eric, our Patterns of Care study
has shown us that the most common chemotherapy
right now in the United States for a
patient like this is dose-dense AC followed
by T, exactly what she received. What are
reasonable alternatives for a patient like
this?
DR WINER: I think any of the so-called
third-generation regimens are reasonable.
I can’t tell you that one is better than the
other, because they haven’t been compared
to each other. The two main regimens are
AC followed by paclitaxel given in a dosedense
fashion, based on the results of the
CALGB Intergroup study (Citron 2003), or
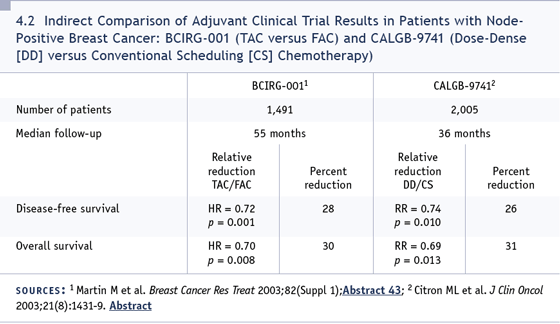
TAC (Martin 2005). I think whatever you’re
most comfortable using as a third-generation regimen is the regimen that you should
use (4.2).
DR LOVE: What about AC followed by
docetaxel? Our Patterns of Care studies show
that is the second most common regimen
used in a case like this.
DR WINER: Why give something that hasn’t
been shown to be effective, although there’s
every reason to think that it will be? Why
not give the regimen as it was given in
a trial? Now, if you have a patient who’s
getting AC followed by paclitaxel and she’s
having severe neuropathy, it’s reasonable to
use docetaxel if you want to continue the
taxane and you think it may be better tolerated
from a neuropathy standpoint.
DR LOVE: Kevin, what regimen would you
use in a patient like this?
DR FOX: We participated in the CALGB-9741
trial and became somewhat familiar with
the dose-dense concept. When that trial
was reported as a positive study, we began
asking, “Why not give dose-dense therapy?”
Gaining more experience with dose density
after the sstudy, we saw no unique toxicities.
It was virtually always assured that patients
could stay on schedule, which trimmed
eight weeks off their course of therapy. Cost
issues of growth factors notwithstanding,
I still haven’t come up with a good reason
not to do it, so it has been our standard
approach outside of a clinical trial.
Select publications
|
